Long term effects of rTMS on the Functional Brain Networks in Treatment-resistant Depression
Ruiyang Ge , Jonathan Downar, Daniel M. Blumberger, Zafiris J. Daskalakis, Raymond Lam & Fidel Vila-Rodriguez
Background
Major Depressive Disorder (MDD)
MDD is a severe disabling and highly prevalent disorder with substantial societal costs. Approximately 60% of the patients do not achieve remission with the initial treatment, and more than 1/3 will eventually be labeled as “treatment resistant”.
Repetitive Transcranial Magnetic Stimulation (rTMS)
- Repetitive transcranial magnetic stimulation (rTMS)2 is a non-invasive, virtually painless neuro-stimulation method. rTMS achieves response rates of 50-55% and remission rates of 30-35% in treatment-resistant depression (TRD) patients.
- Recent work has placed rTMS as a first line option for TRD, yet its mechanism of action largely remains to be elucidated.
Potential Neural underpinnings of rTMS on MDD
- The dorsolateral prefrontal cortex (DLPFC)2 has been the primary area of interest for rTMS stimulation, and accumulating evidence has demonstrated its therapeutic role in treating MDD.
- The anterior cingulate cortex (ACC), especially its subgenual portion (sgACC)4, has been consistently found to be hyperactive in MDD patients, and the normalization of sgACC hyperactivity is associated with MDD remission.
- MDD results from the dysfunction of networks of brain regions5. The rTMS treatment applied to the stimulation site induces changes both locally and in distantly interconnected regions4,6, thereby modulating functional connections of brain networks.
In the present study, we measured functional connectivity of the intrinsic brain networks in TRD patients initiating a 4-6 week course of rTMS treatment, with the primary aim to detect intra-network and inter-network changes reflecting rTMS-induced improvements in depressive symptoms.
Methods
Patients initially received 20 sessions of rTMS once daily, 5 days/week, with extension to 30 sessions for those with at least 30% improvement in depressive symptoms. Two MRI scans were measured at baseline and at the follow-up session (12 weeks after the final treatment session). For healthy controls (HCs), the follow-up data were collected 18 weeks post baseline.
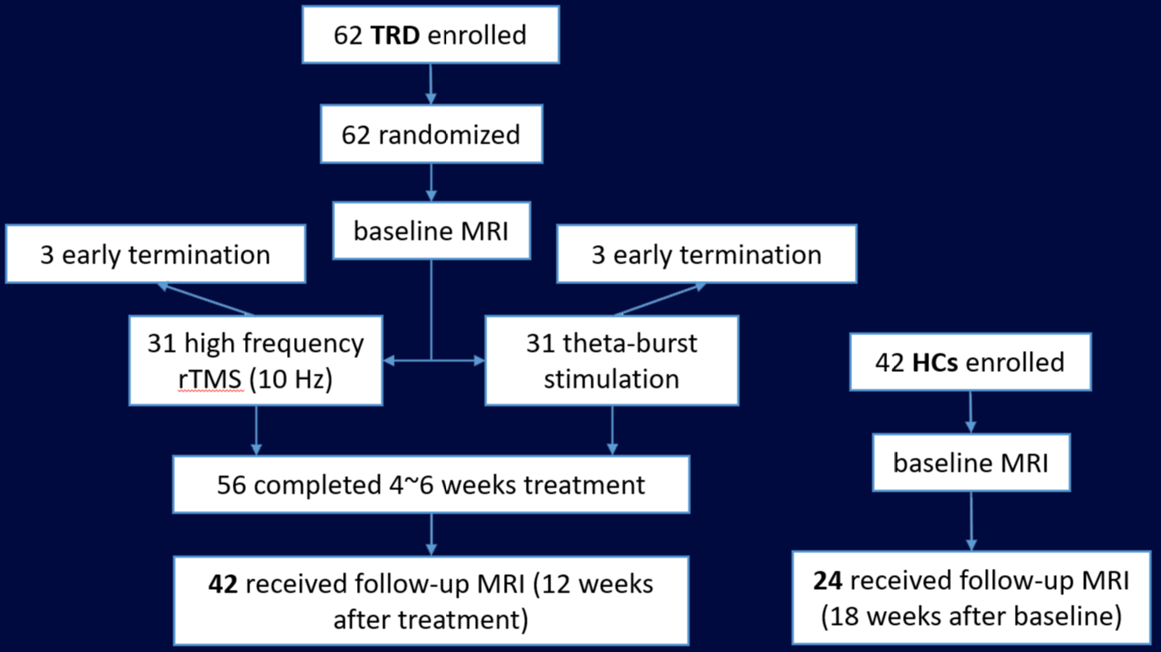
We analyzed resting-state functional MRI (fMRI) from 42 TRD patients and 24 healthy controls (HCs) scanned at UBC (Table 1). We applied a new group independent component analysis approach7 to the fMRI data of all subjects to estimate their intrinsic networks.
Table 1. Sample demographics and clinical characteristics for the two comparison groups
| Characteristic | TRD patients, Mean (SD) (n = 42) | Healthy controls, Mean (SD) (n = 24) | p value |
| Age | 42.86 (12.32) | 45.25 (12.19) | 0.45a |
| Sex (F/M) | 24/18 | 12/12 | 0.58b |
| Education, y | 15.38 (2.15) | 16.50 (2.23) | 0.05a |
| Age at onset, y | 25.62 (11.97) | – | – |
| Duration of current episode, mo. | 37.93 (44.64) | – | – |
| HDRS score, baseline | 21.71 (3.92) | – | – |
| HDRS score, end of treatment | 10.00 (6.65) | – | – |
| HDRS score, follow-up | 10.57 (7.71) | – | – |
Results and Summary
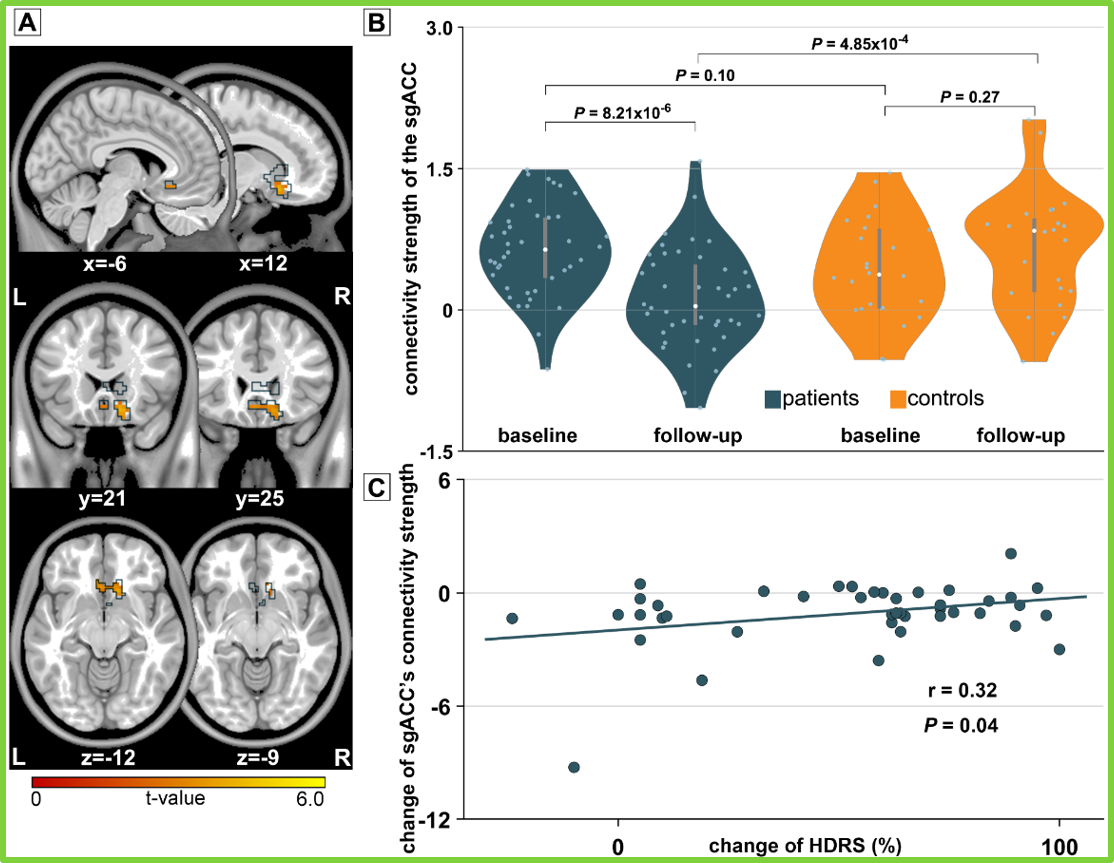
Figure 2 vaDMN: ventral-anterior default mode network; vpDMN: ventral-posterior DMN; daDMN: dorsal-anterior DMN; dpDMN: dorsal-posterior DMN; rCEN: right central executive network; lCEN: left central executive network.
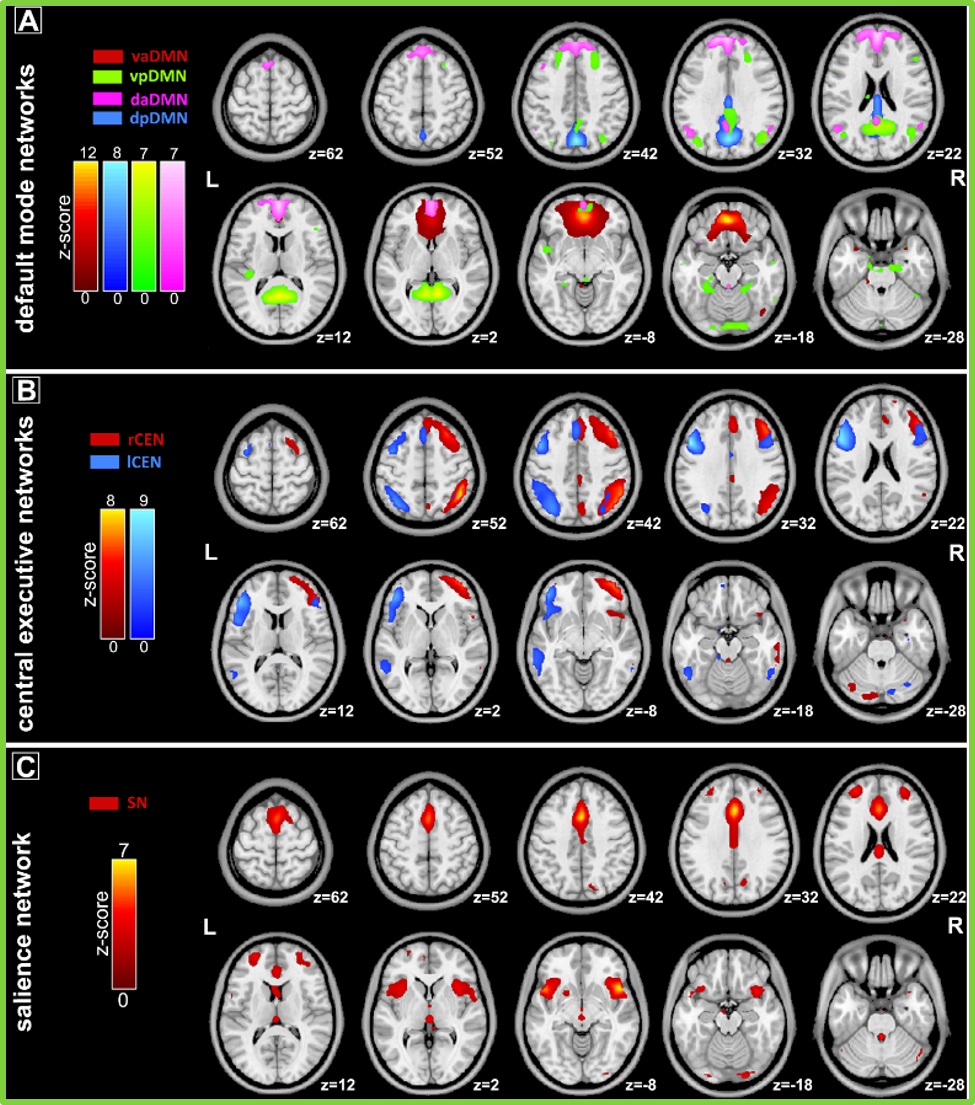
Figure 1.
- Fig.1: Large-scale intrinsic brain networks identified using independent component analysis (ICA). Data from 42 TRD patients and 24 healthy control subjects were combined in a group ICA to identify 39 independent components (networks) across all participants and all scans in a data-driven manner. Seven of these components correspond to coronial functional networks.
- Fig.2: Changes of connectivity strength of the subgenual anterior cingulate cortex (sgACC) cluster. (A, B) Changes of connectivity strength of the sgACC cluster in the ventral-anterior default mode network (vaDMN) of the patient and healthy control samples. The result was masked with the corresponding component mask generated from significant time × group interaction (panel A). (C) Scatterplot depicts the positive correlation of the changes of the sgACC connectivity strength and changes of Hamilton Depression Rating Scale (HDRS) scores.
- Fig.3: No significant differences of the inter-network interactions between the seven networks of interest were found between patients and healthy controls as well as between baseline and follow-up scans.
- Fig.4: Path modeling result indicated a significant indirect effect was observed, indicating that decrease of the sgACC connectivity mediated an effect of vaDMN-rCEN interaction changes on the symptom relief.
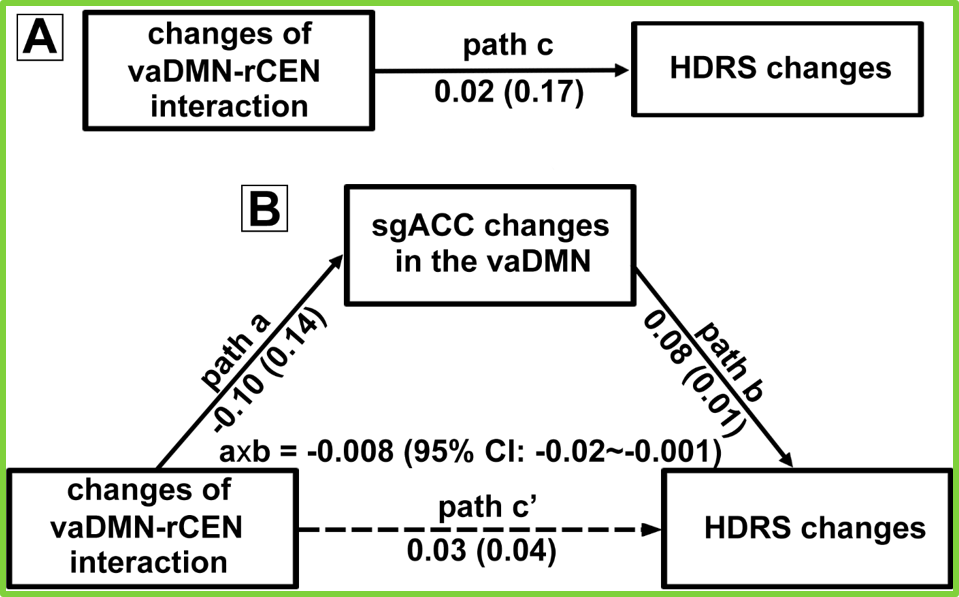
Figure 4
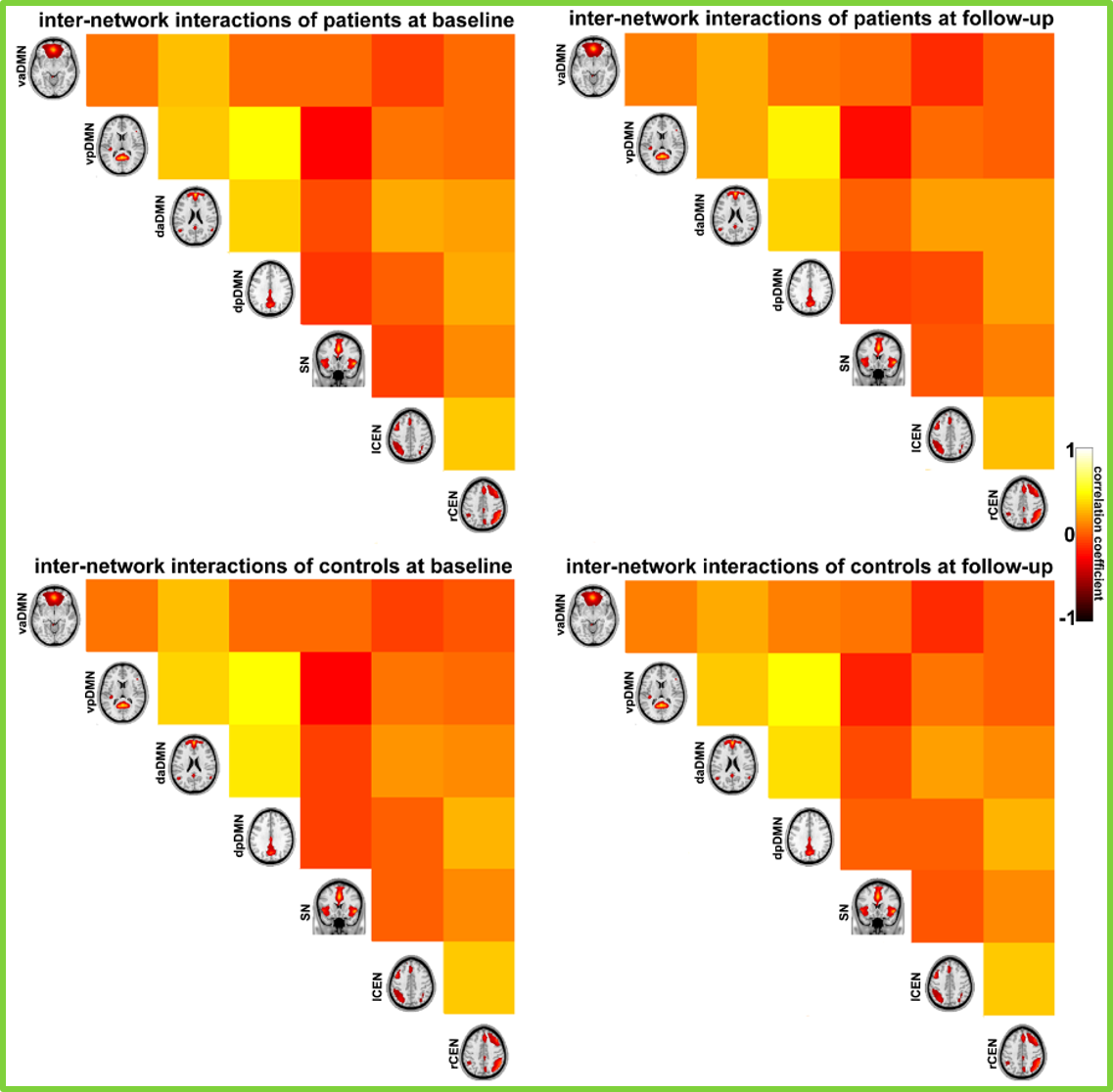
Figure 3
- We additionally demonstrated that the decrease of the sgACC connectivity strength with rTMS in a 3-month term was not driven by using medication, different treatment protocols. Also, the decrease of the sgACC connectivity strength with rTMS were only detected in patients who did not change their response status between treatment end and 3-month follow-up sessions.
- The current results provide a neurobiological support for the notion that the antidepressant effects of rTMS are mediated through changes in interactions between brain networks, and provide direct evidence with the sgACC connectivity as a therapeutic neuro-modulation target for TRD.
References
1. RH Belmaker, et al., New England Journal of Medicine, 2008
2. JP O’Reardon, et al., Biological Psychiatry, 2007
3. RV Milev, et al., Canadian Journal of Psychiatry, 2016
4. MD Fox, et al., Biological Psychiatry, 2012
5. RH Kaiser, et al., JAMA Psychiatry, 2015
6. NS Philip, et al., Biological Psychiatry, 2018
7. R. Ge, et al., NeuroImage, 2015